Making chemical solutions
An important part of Chemistry is the ability to make up solutions of a known concentration. This can sound quite complex, however it doesn't need to be.
The following is some simple instructions that will produce 250m of 1molar concentration of Sodium Chloride
I have made this solution up using pure Sodium Chloride, from a chemical supplier [1], rather than table salt which contains Sodium Ferrocyanide. If you need extra help with this try asking on Science forums [2] as there is a section on there for home chemistry.
I am also on the IRCNow network, where we are starting up a channel to discuss amateur / home science.
Links
1 Better Equipped
2 Science Forums
3 Amateur Science
Tags
#Chemistry,#NaCl,#SodiumChloride,#Molar,#Mol,#Solution,
AI statement : Consent is NOT granted to use the content of this blog for the purposes of AI training or similar activity. Consent CANNOT be assumed, it has to be granted.

Molecular weight calculator
I decided to write this to help calculate molar weights for chemistry.
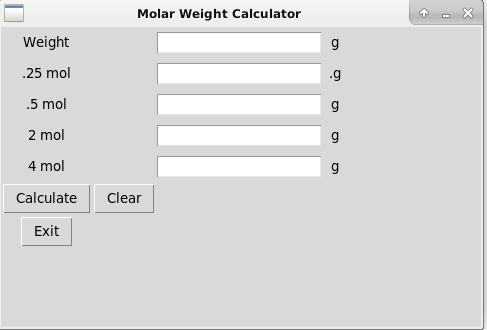
In essence you can enter the Mass of an element or molecular mass of a substance, compound etc, and this will help give you molar weight, for example 0.5 mol.
It is, for example common to have different concentrations of an acid for example. We know that Sodium Hydroxide has a weight of 40 because of the combined weight of its components:
$NaOH$ which equates to
Na = 22 +
O = 16 +
H = 1
= 39
Therefore 1 mol of NaOH = 39g which is of course equal to Avogadros constant : $6.022 x 10^{23}$
Therefore 0.5 mol is roughly $39 \div 2 = 19.5g$
This program is NOT a substitute for proper calculation. You need to use more accurate values. Values used are just a rough guide.
However it may be useful, for those quick calculations.
The program code base is taken from my Drake equation calculator I made a few weeks ago.
#chemistry,#mol,#molar,#weight,#calculator
AI statement : Consent is NOT granted to use the content of this blog for the purposes of AI training or similar activity. Consent CANNOT be assumed, it has to be granted.

